Regulatory bodies yet to approve HIV self-testing kit
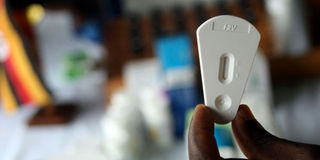
An approved HIV testing method: the Erisa system. There are contradictions on the approval and validation of a new home testing kit, OraQuick. PHOTO | FILE | NATION MEDIA GROUP
What you need to know:
- While the National Aids and STI Control Programme has indicated that the recently launched OraQuick has been certified by three government agencies, investigations by the Nation revealed that the kits are yet to get approval.
- OraQuick is an oral swab test that does not require blood samples.
Use of the newly launched oral HIV self-testing kit has come into question after it emerged that Kenya’s regulatory authorities are yet to fully approve the technology.
While the National Aids and STI Control Programme (Nascop) has indicated that the recently launched OraQuick has been certified by three government agencies, investigations by the Nation revealed that the kits are yet to get approval.
Validation is the scientific process of verifying the fitness-of-purpose for specific test devices for disease detection, monitoring and/or surveillance in a particular geographical setting.
According to its manufacturer, Orasure Technologies Inc, which is based in the US, OraQuick is an oral swab test that does not require blood samples.
TEST TAKES 20 MINUTES
The manufacturer explains in its website that the test takes just 20 minutes “in the comfort and privacy of your own home.”
After South Africa, Kenya is the second country in Africa to introduce the device.
Anjiwa Company Ltd is the local distributor. According to the head of Nascop, Dr Martin Sirengo, the oral kit has been validated by the Pharmacy and Poisons Board, Kenya Medical Laboratory Technicians and Technologists Board and National Public Health Laboratory Services.
A subsequent statement by Nascop’s communications department further affirmed that the approval had been conducted by relevant regulators.
LISTED PRODUCT
But in separate interviews, all the three government agencies mentioned by Nascop denied involvement in validating the self-test kit.
The Pharmacy and Poisons Board acting chief executive officer Fred Sioyi (also its acting registrar) said the board had only listed the product. Dr Sioyi said the mandate of validating such products lies with the Medical and Technicians board.
“Validation is done by the Medical Board. The Pharmacy Board does listing. We have listed the product,” said Dr Sioyi.
Mr Mamo Umuro, head of the Division of National Public Health Laboratory Services at the Ministry of Health also denied that his agency had issued a statement.
“There is no validation certificate that we issued that I am aware of,” said Mr Umuro.
VALIDATION CERTIFICATES
He added: “From our side, we do not issue validation certificates. The people who issue certificates are the Medical Board, not even the Pharmacy Board. These are lab products; it’s for the Medical Board. For us, if a product is brought to us for validation, we validate and give the report back to the Medical Board. By law, we don’t offer any validation certificate.”
Medical Board chairman Abel Onyango, while maintaining that the board is the sole agency mandated to validate and register all invitro diagnostics in Kenya, said OraQuick HIV kit cannot be traced to their data base.
“As a board, we recognise Anjiwa Company as a distributor of invitro diagnostics but the validation records for OraQuick HIV test kit is missing in our system.
However, we are investigating how the device could have entered the market without following the right procedures,” said Mr Onyango.
IMPORTED KIT
Experts say the test performance of an imported disease testing kit may be influenced by various factors — human, environmental, temperature, humidity and burden of disease or prevalence in a particular setting.
When contacted, Mr Jilani Yawa, the local representative of Anjiwa Company, refused to be drawn into discussions on whether OraQuick was validated before roll-out and insisted that we establish the same from Nascop.
“Just go and discuss that with Nascop,” he said, when we made a telephone call to him. Nascop head Sirengo took part in the launch of the product in Kenya last month.
The kit sells at between Sh700 and Sh800 in selected drug outlets, and claims it has over 80 per cent accuracy in detecting HIV in oral fluid.
HIV STATUS
Millions of Kenyans have been advised to establish their HIV status so as to take precautionary measures.
According to the Ministry of Health, 90 per cent of Kenyans need to know their HIV status.
A further 90 per cent of those with known status need to be put on treatment. The OraQuick kit is expected to promote home-testing to reduce visits to VCTs and clinics.
DISEASE DIAGNOSIS
Before any kit is introduced to Kenya, care must be taken to safeguard and protect the public through ensuring that all medical laboratory equipment and reagents that are manufactured, imported, distributed and used for disease diagnosis meet the set quality and safety standards.
Mr Onyango said validation is important as it verifies the kit’s test performance and avoids litigations arising from misdiagnosis.
The findings come even as a recent report shows that truck drivers and sex workers, one of the key populations at risk of contracting HIV, have embraced the self-administered self-testing kit.





