How a mutation to fight malaria came back to bite us
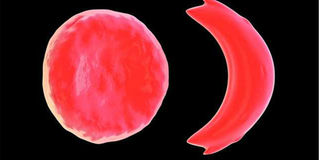
Normal red blood cell (left) and a sickle cell (right). PHOTO| FILE
Thousands of years ago, in what is now the Sahara Desert, a child was born with heightened immunity to malaria.
This was important because at the time, that part of Africa was wet and covered by a dense forest – just the right habitat for malaria-carrying mosquitoes.
With a better chance against an illness that was a major killer, the child lived into adulthood and had children. Those children had extra defences against malaria that helped them live longer, defences their descendants around the world still have today, more than 250 generations later, according to a study published in March in the American Journal of Human Genetics.
After analysing the genetic material of nearly 3,000 people, 156 of whom had sickle cell disease, and tracing the mutation 7,300 years back, the researchers found it started with just that one child. Back then, this genetic mutation was a good thing, because it made people more resistant to malaria, but it came with consequences: The sickle cell trait.
Normally, red blood cells deliver oxygen throughout the body, thanks to haemoglobin, bending and adjusting to fit into narrow blood vessels, to deliver oxygenated blood to tissues and organs. On the other hand, rigid and misshapen sickle cells with abnormal haemoglobin, get stuck in the narrow blood vessels as they try to push through. This prevents blood with oxygen from reaching tissues and organs, causing anaemia that leaves the patient tired and out of breath and sudden attacks of severe pain called sickle cell crises.
Moreover, without adequate oxygenated blood, tissues and organs such as the spleen, liver and kidneys suffer damage over the years, with potentially life-threatening results.
With each sickling cycle, the cells accumulate damage, becoming denser than normal red blood cells. And complications become more likely as the patient gets older and symptoms persist.
“For children, the biggest concerns are pain and a higher risk of infection as a result of a compromised immune system from an enlarged spleen,” explains Dr Kibet Shikuku, a specialist in diseases related to blood (haematologist).
About five per cent of the world’s population carries trait genes for haemoglobin disorders, mainly, sickle cell disease and thalassaemia. The former is particularly common among people whose ancestors come from sub-Saharan Africa, India, Saudi Arabia and Mediterranean countries.

Pupils and students from schools in Mombasa during a procession to mark the World Sickle Cell Day on June 19. Counties at the coast and western Kenya account for 20 to 30 per cent of all sickle cell disease patients in Kenya. PHOTO| GIDEON MAUNDU| NATION
Sickle cell disease is most common in Africa, and affects up to two per cent of the population in tropical countries such as Cameroon, according to the World Health Organisation (WHO).
A recent study by the WHO and Masinde Muliro University of Science and Technology showed that 20 to 30 per cent of all patients with sickle cell disease are in counties in western Kenya and the coast.
According to Dr Shikuku, who works at Kenyatta National Hospital, diagnosis for sickle cell disease varies from one individual to another due to differences in the blood composition of individuals.
“Blood has a lot of elements, one of them being globin which, in simple terms, we refer to as chains,” he explains.
“During conception, both parents contribute these chains that form the composition of the haemoglobin (an iron-containing protein in blood that carries oxygen from the lungs to body tissues). Parents contribute an alpha chain and a beta chain.”
However, he says, there is formation of a third chain called the foetal chain, found in infants. The beta chain, responsible for the sickle cell trait, if there is a mutation in the beta-globin gene, is usually suppressed by foetal chains, which some children retain years after being born, making it difficult to detect the condition. However, as soon as the foetal chains are lost, the trait begins to manifest.
Dr Shikuku adds that when red blood cells are malformed, the person could be either a carrier of the disease or a patient, depending on the degree of the malformation.

Sickled cells and normal red blood cells. With each sickling cycle, the cells accumulate damage, becoming denser than normal red blood cells. PHOTO| FILE
Despite growing awareness on the disease, Kenya is far from eliminating the myths and misconceptions around sickle cell disease. Even worse, many patients die out of frustration and inability to manage the disease.
The main symptoms are pain attacks, or crises, which can last up to a week, susceptibility to infections, and anaemia, which causes weakness and fatigue. The pain can strike at any time, without warning.
However, not all patients suffer from painful crises. This, according to Dr Shikuku, is because different people react differently to the condition.
“As an individual, you cannot prevent the blockage in the veins, but you can reduce the number of obstructions by avoiding the causative agents.
“When sickle cells have adequate supply of oxygen, they behave like normal cells, hence there is no pain. However, a slight depletion of oxygen leads to formation of the crescent shape which blocks the veins and results in painful episodes,” he explains.
Most children with the most severe form of sickle cell disease die before the age of five, according to the WHO.
Just 30 years ago, children with sickle cell disease often died at a very young age, but advances in treatment have improved quality of life and expected lifespan for many. Patients now live well beyond the age limit of 18.
A bone marrow transplant is the only known cure for sickle cell disease, but finding a well-matched donor is not easy. Therefore, only one out of 10 patients benefit from this option.
Many of the rest are put on hydroxyurea, a medication used to decrease the number of attacks by increasing foetal haemoglobin and the volume of red blood cells and their haemoglobin levels.
But a breakthrough is within reach: last year the US Food and Drug Administration approved a new drug, Endari (also called L-glutamine oral powder), to help reduce complications associated with sickle cell disease.
The drug is the first treatment approved for patients with sickle cell disease in almost 20 years, and is recommended for patients from the age of five years.
***
Treatments in the pipeline
A key signalling protein that regulates haemoglobin production in red blood cells, could offer a possible target for a future innovative drug to treat sickle cell disease (SCD).
Blocking the protein reduces sickling. The signalling protein HRI, regulates the production of haemoglobin and plays a role in haemoglobin switching, a transition in newborns when red blood cells switch from a foetal form to an adult form.
The mutation that causes SCD is present in the adult form of haemoglobin. SCD patients with higher ratios of foetal haemoglobin than adult haemoglobin have a milder form of the disease.
The drug hydroxyurea, which increases foetal haemoglobin is not effective in all patients. HRI helps to silence foetal haemoglobin production in adult red blood cells. When HRI function was selectively knocked out during the study, levels of of foetal haemoglobin increased. Researchers were able to decrease sickling in red blood cells from SCD patients, without impairing the viability or maturation of the cells.
When they combined HRI depletion with treatment with pomalidomide, an experimental drug known to increase foetal hemoglobin, the two had a stronger effect than when used separately.
The findings were published in the journal Science this July



